Explore Our Comprehensive Cycle of Cultivation, Production, Processing, and Manufacturing Before Partnering

Our Transparent Commitment to
Care from Farm to Product

The Raw Herbs
Authentication-The starting of the process of the raw material for preparation of a medicament should be authenticated on the basis of Botanical (Pharmacognostic) characters.



Organoleptic
Sensory evaluation & other morphological characteristics -To be matched with standard reference. Visual characters like size, colour, surface characteristics like texture and fracture characteristics and other characters like odour and taste. The size of the plant material may be used as an identification character. The colour of the material may be compared with an authentic material for genuineness. The odour of the plants may be a characteristic feature.



Washing process


Sorting
Sorting

Preliminary
Washing
Preliminary Washing

Final
Washing
Final Washing

Three RO/DM washings to the ingredient to eliminate any further foreign matter & final rinsing is given separately with RO/DM water.
Drying
Drying

Water is soaked in layered muslin cloth from washed herbs with slight compression & kept in rectangular SS trays in a hot air oven at 45°C.

Filteration Process

A. Coarse Powder (10/44): A powder of which all the particles pass through a no. 10 sieve & not more than 40% through no.44 sieve.
B. Moderately Coarse Powder (22/60): A powder of which all the particles pass through a no. 22 sieve & not more than 40% through no.60 sieve.
C. Moderately Fine Powder (44/85): A powder of which all the particles pass through a no. 44 sieve & not more than 40% through no.85 sieve.
D. Fine Powder (85): A powder of which all the particles pass through a no. 85 sieve.
E. Very Fine Powder (120): A powder of which all the particles pass through a no. 120 sieve.
Unfiltered material is again subjected to grind & filter as above.

Mixing
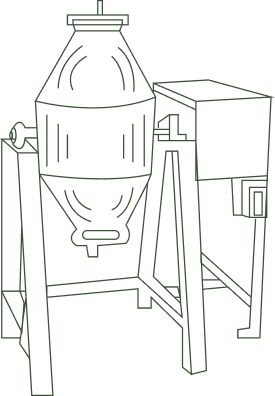

Mixing
Time of mixing: 2 Hours; one hour clockwise direction & one hour anti-clockwise direction


Quality Control
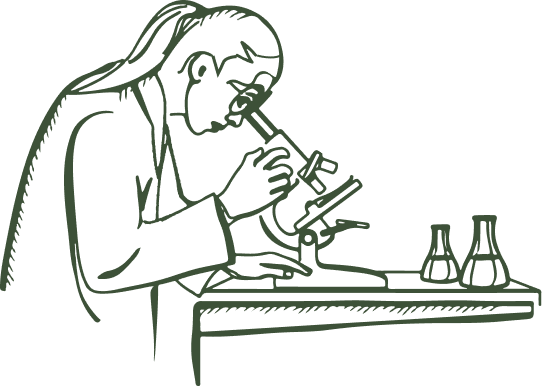
Quality Control
Quality control measures to comply: Powder
- Analysis Status: Should Comply as per pharmacopeia: Herb Fingerprinting
- Loss on Drying
- Total Ash
- Acid-insoluble ash
- Extractable value (Alcohol and Water)
6. Heavy Metals (Lead, Cadmium, Mercury & Arsenic) - As prescribed in respective Pharmacopeias.
7. Microbial Load - The limits as prescribed in respective Pharmacopeias. i.e. Staphylococcus aureus/g.- absent, Salmonella sp./g.- absent, Pseudomonas aeruginosa/%- absent, Escherichia coli- absent, Total Microbial plate count (TPC) & Total Yeast & Mold- As prescribed in respective Pharmacopeias.
8. Aflatoxins (Bi, B2, Gi, G2) - The limits as prescribed in respective Pharmacopeias.
9. Chromatographic profiles (TLC, HPLC or GC)
10. Assay of Marker Compounds
11. Pesticide Residue (OrganoChlorine and Organophosphorus)- The limits as prescribed in respective Pharmacopeias.


End Product


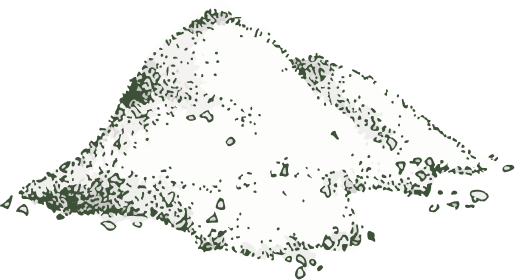

After undergoing the entire process, the end product derived from these procedures serves various purposes based on specific requirements. It finds applications in herb marking, capsule production, and formulation development.